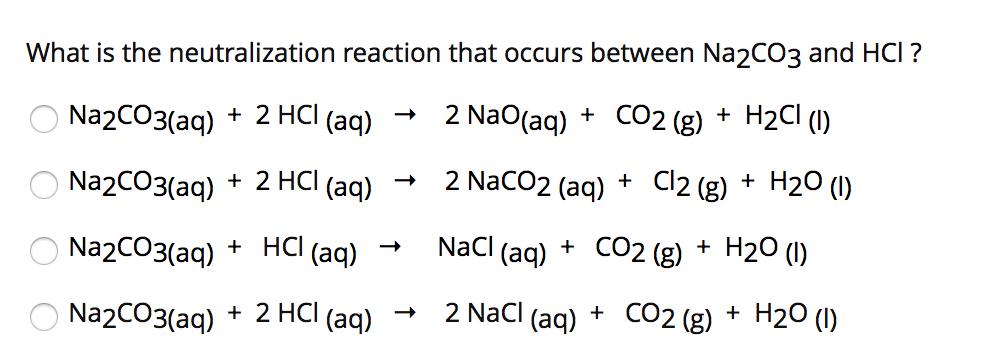Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid | Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Hello, Chemistry Enthusiasts! For today's
in the titration of Na2CO3 by HCl using methyl orange indicator, thevolume required at the equivalence point will be if that of the acidrequired using phenolphthalein indicator is 10.0 ml:

give inference for following test :- mixture+dil Hcl pass the evolved gas into NaCo3 phenolphthalein reagent - Brainly.in

We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com

Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +
What is the pH reading after the endpoint of the neutralization reaction of calcium carbonate and HCL? - Quora

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa

Question Video: Determining the Products Formed from the Reaction between Sodium Carbonate and Hydrochloric Acid | Nagwa

SOLVED: 2. Na2CO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:
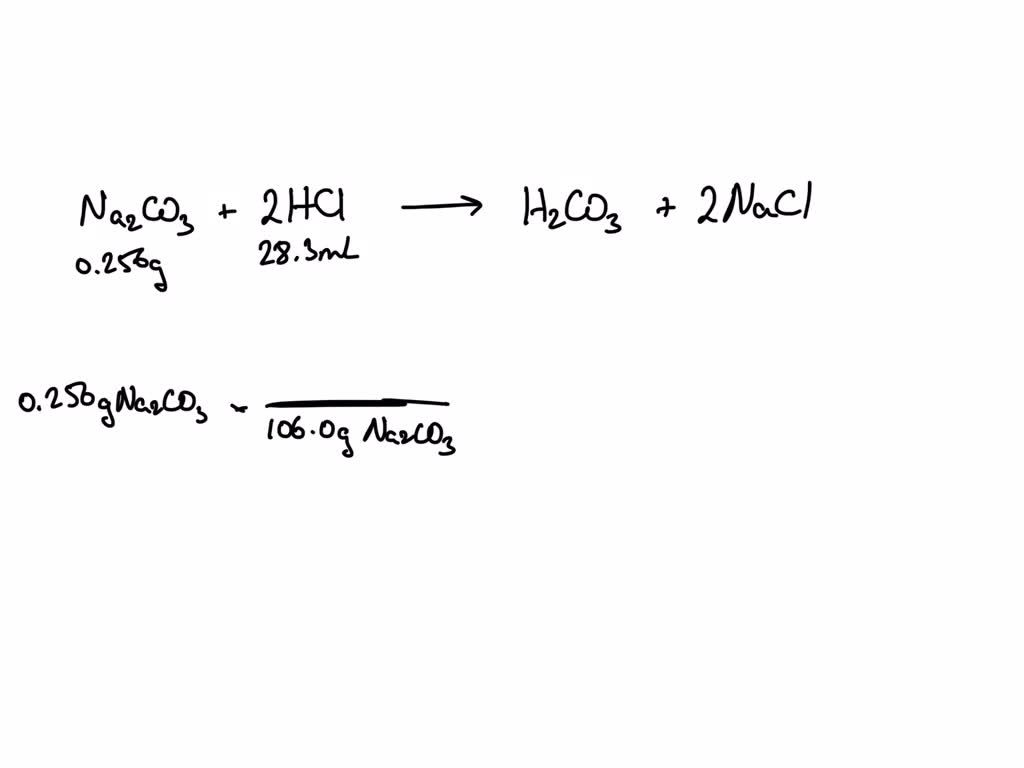
SOLVED: What is the molarity of a HCl solution if 28.3 mL of the solution are required to react with 0.256 g of the Na2CO3?

In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with phenolphthalein indicator and y mL with methyl orange indicator in the same titration. Hence, volume for complete




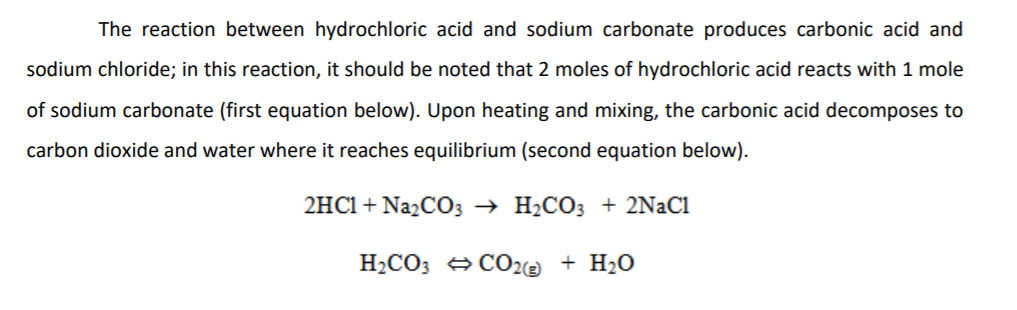



![ANSWERED] Sodium carbonate and hydrochloric acid re... - Physical Chemistry ANSWERED] Sodium carbonate and hydrochloric acid re... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/51622204-1659180079.3710194.jpeg)