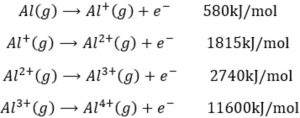Difference Between First and Second Ionization Energy (I1E vs I2E) | Compare the Difference Between Similar Terms
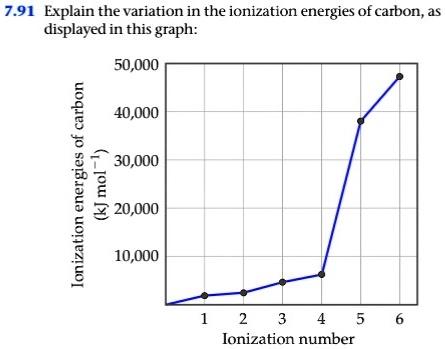
SOLVED: 7.91 Explain the variation in the ionization energies Of carbon displayed in this graph: 50,000 1 40,000 30,000 1 1 220,000 7 10,000 2 4 5 Ionization number

The first ionization energy of carbon is more than boron, but the second ionization energy is in reverse. Why? - Quora

Lesson objectives Define first ionisation energy and successive ionisation energy. Explain the factors that influence ionisation energies. Predict the. - ppt download

Premium Vector | Carbon chemical element with first ionization energy atomic mass and electronegativity values on scientific background

The correct order of the second ionisation potential of carbon, nitrogen, oxygen and fluorine is - YouTube