Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.

The standard heat of formation of carbon disulphide (l) given that standard heat of combustion of - YouTube

The combustion enthalpies of carbon, hydrogen and methane are 395.5 kJ/mol , - 285.8 kJ/mol and - 890.4kJ/mol respectively at 25^∘C . The value of standard formation enthalpies of methane at that temperature is:
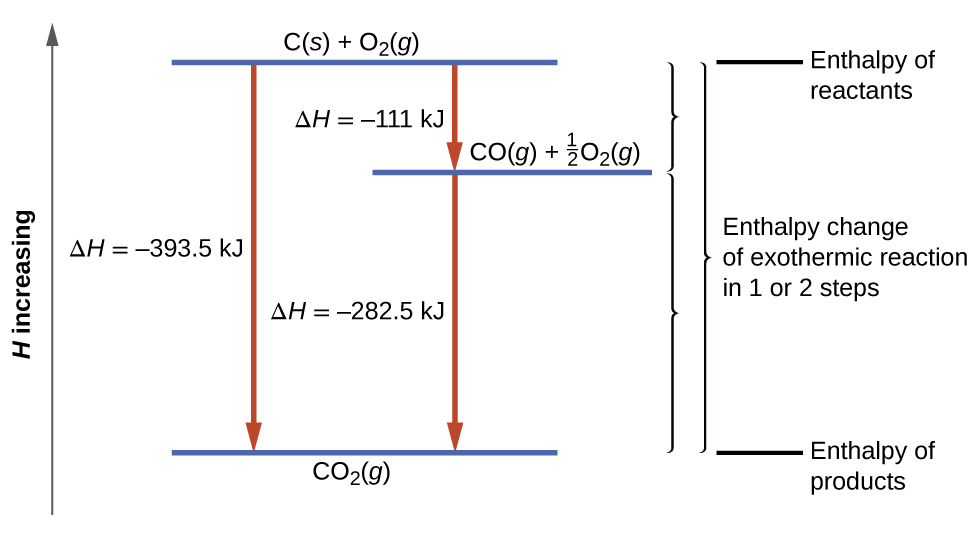
What is the enthalpy of formation of carbon monoxide in KJ/mol ? C(s) + O2(g) --> CO2(g) ΔH° = -393 kJ 2CO(g) + O2(g) --> 2CO(g) ΔH° = -588 kJ | Socratic
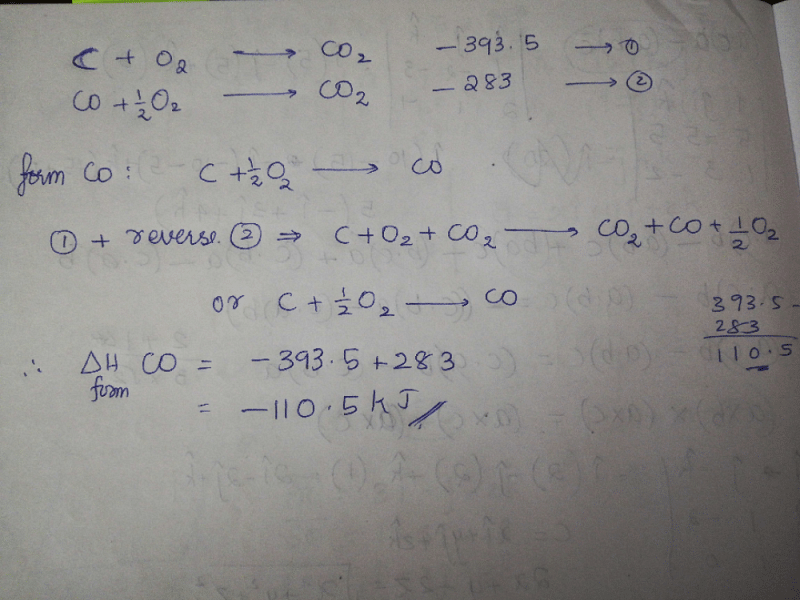
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 KJ mol¹ respectively .The enthalpy of formation of carbon monoxide per mol isa)-110.5 KJb)-676.5 KJc)+676.5 KJd)110.5 KJCorrect answer is

The enthalpies of combustion of carbon and carbon monoxide are - 393.5 KJ and - 283 KJ respectively the enthalpy of formation of carbon monoxide is :

41524992Given standard enthalpy of formation of `CO(-110 \"KJ mol\"^(-1))` and `CO_(2)(-394 \"KJ mol - YouTube

SOLVED:The enthalpy of combustion of solid carbon to form carbon dioxide is -393.7 kJ / mol carbon, and the enthalpy of combustion of carbon monoxide to form carbon dioxide is -283.3 kJ /
The combustion enthalpies of carbon, hydrogen, and methane are 395.5 kJ mol^ 1, 284.8 kJ mol^ 1 and 890.4 kJ mol^ 1 respectively at 25^0C. The value of s†an dard formation enthalpies

Calculate the standard heat of formation of carbon disulphide (l). Given that the standard heats of - YouTube
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and –283kJ mol^–1 respectively. - Sarthaks eConnect | Largest Online Education Community

The enthalpy of combustion of methane, graphite and dihydrogen at 298K are - 890.3 kJmol^-1, - 393.5 kJmol^-1, - 285.8 kJ mol^-1 respectively. Enthalpy of formation of CH4(g) will be:

The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:

The enthalpies of combustion of carbon and carbon monoxide are `-390 kJ mol^(-1)` and `-278 kJ mo - YouTube
![Enthalpy of combustion of carbon to CO2 is - 393.5 kJmol^-1 . The heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas is: [Molar mass of CO2 = 44 gmol^-1 ] Enthalpy of combustion of carbon to CO2 is - 393.5 kJmol^-1 . The heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas is: [Molar mass of CO2 = 44 gmol^-1 ]](https://haygot.s3.amazonaws.com/questions/1961395_1291143_ans_d6642064cda84dfe904274b61e90a2dc.jpg)
Enthalpy of combustion of carbon to CO2 is - 393.5 kJmol^-1 . The heat released upon formation of 35.2 g of CO2 from carbon and dioxygen gas is: [Molar mass of CO2 = 44 gmol^-1 ]

The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is: