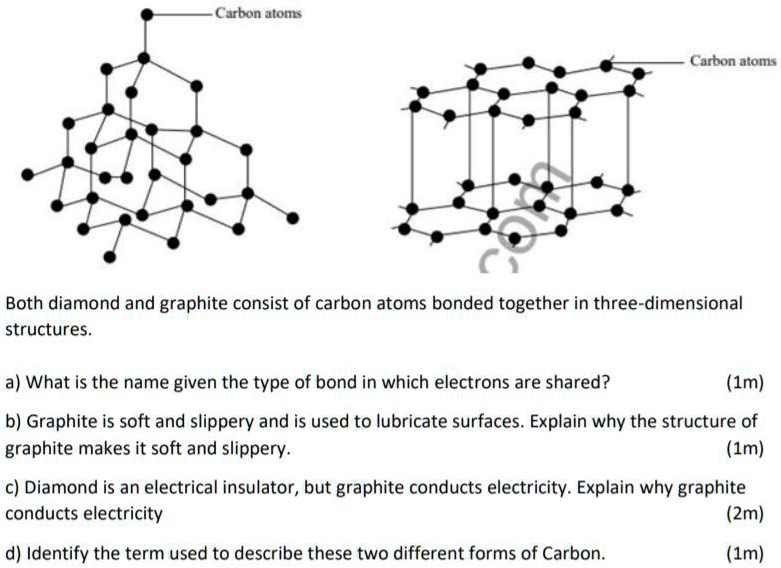
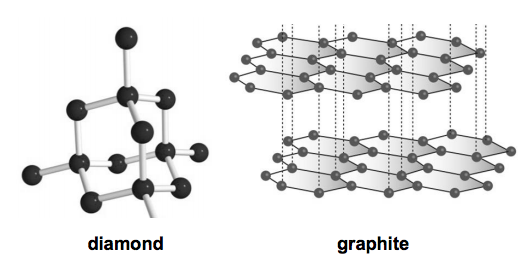
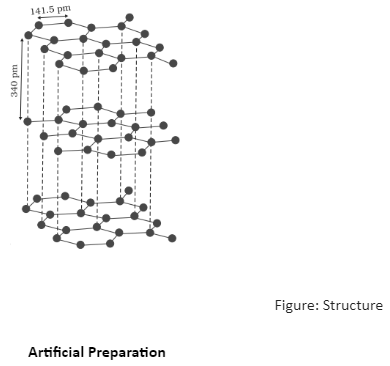
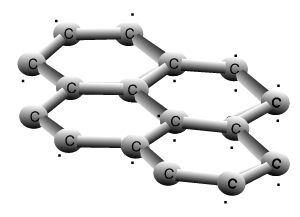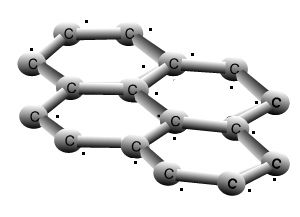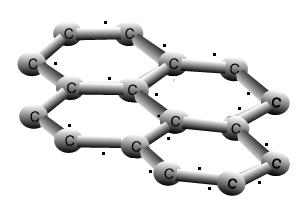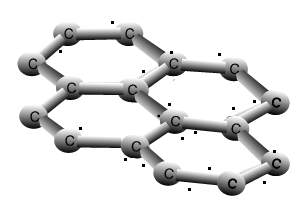
Why is graphite a good conductor of electricity and a good lubricant? Explain with the help of diagram.

How does graphite conduct electricity even if it is bonded with three carbon atoms in which one of the bond is double, therefore it is chemically stable? How does free electron come

It is given that graphite has 2 single bonds and 1 double bond( in the pic) So why does graphite - Science - Carbon and its Compounds - 12375167 | Meritnation.com
How does graphite conduct electricity even if it is bonded with three carbon atoms in which one of the bond is double, therefore it is chemically stable? How does free electron come

we know that graphite conducts electricity as it has free electrons but it says valency of carbon is satisfied due - Science - Carbon and its Compounds - 11116453 | Meritnation.com

Why is graphite a conductor of electricity - Science - Carbon and its Compounds - 14532383 | Meritnation.com