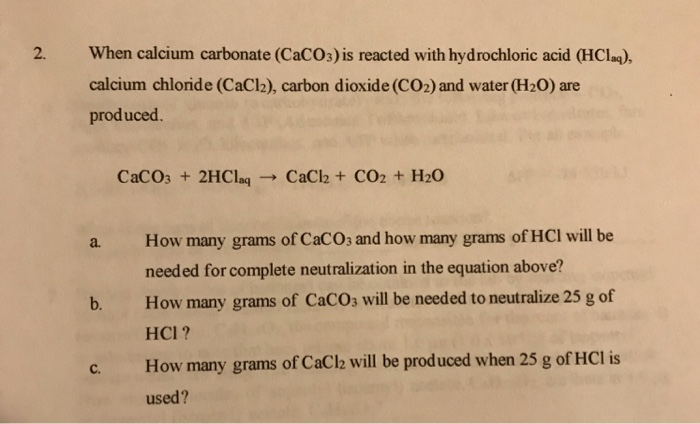Preparation of SMA/CaCO3/CaCl2@SiO2 as a Fluid Loss Agent Based on PSA/Ca-MMT/CaCl2@SiO2 | American Laboratory

Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction - Chemistry - Some Basic Concepts of Chemistry - 16839929 | Meritnation.com
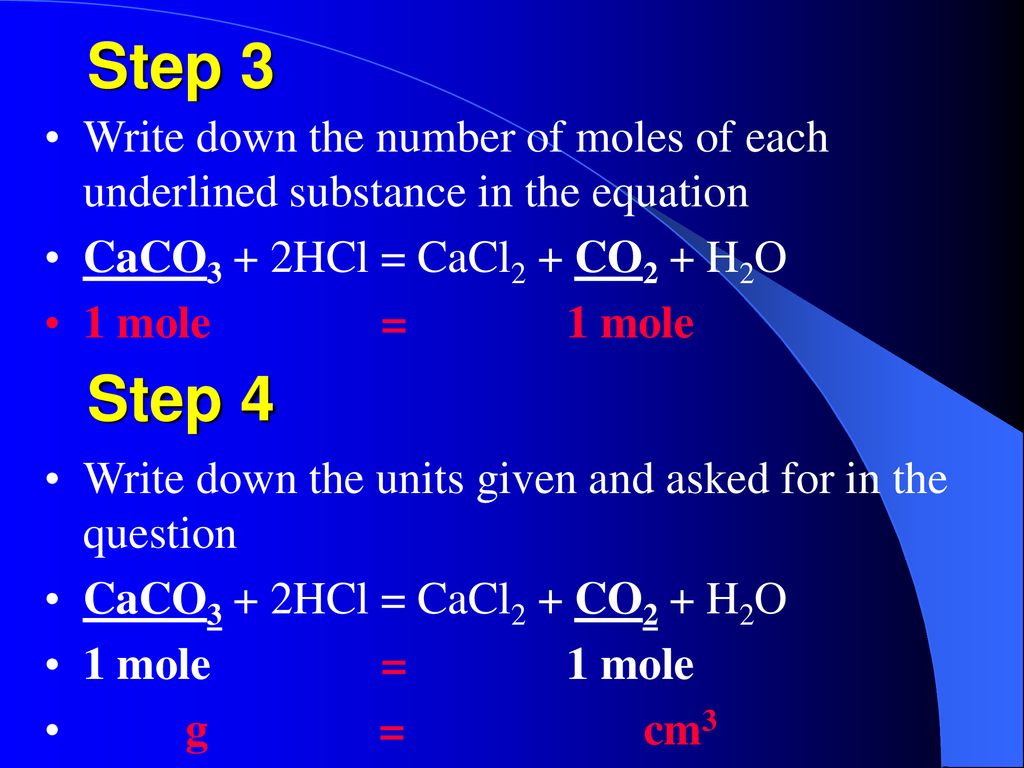
The mass of CaCO3 required to react completely with 20 mL of 1.0 M HCL as per the reaction CaCO3+2HCl–>CaCl2+CO2+H2O
16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3

SOLVED: If 2.5g of CaCO3 is mixed with 2.0 g of HCl to complete the reaction CaCO3 + 2HCl ——–> CaCl2 +CO2 +H2O, What is limiting and what amount of CO2 will

SOLVED: For the reaction CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l). Calculate the mass of CaCl2 that can be obtained if 115 g of CaCO3 and 76.72 g of HCl resulted in a 100% yield of CaCl2. Molar
Preparation of Ultra-fine Calcium Carbonate by a Solvent-free Reaction using Supersonic Airflow and Low Temperatures

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
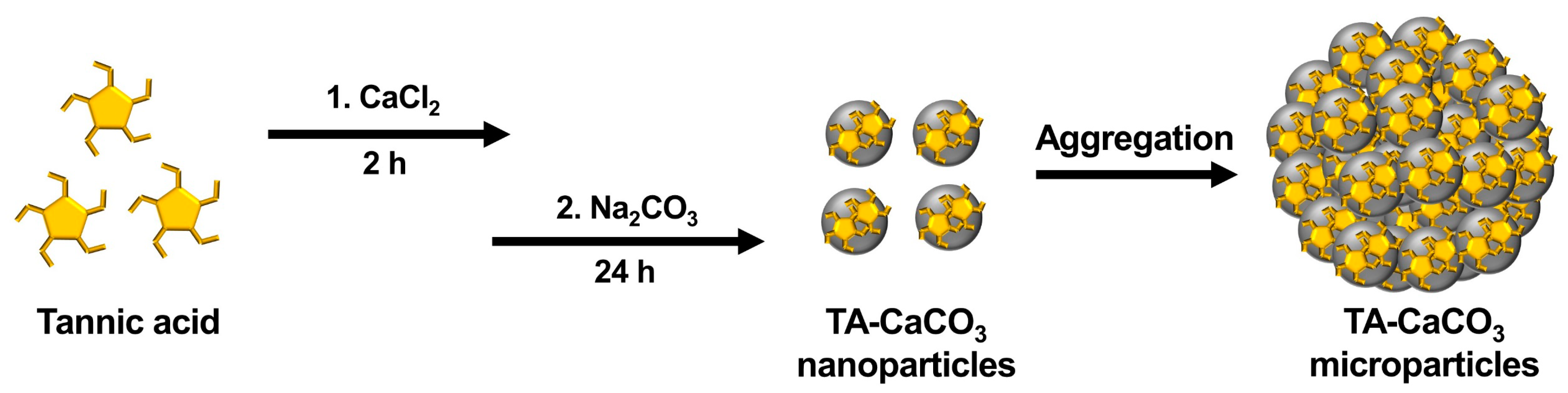
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects

HCl+CaCO3=CaCl2+H2O+CO2 balance the chemical equation @mydocumentary838. hcl+caco3=cacl2+h2o+co2 - YouTube

Influence of salts concentrations on CaCO3 particles formation: SEM... | Download Scientific Diagram

Q2. calcium Carbonate Reacts With Aqueous Hcl To Give Cacl2 And Co2 According To The Reaction Given Below: Caco3 (s) + 2hcl (aq) → Cacl2(aq) + Co2(g) + H2o(l) What Mass Of


![ANSWERED] Na₂CO3(aq) +CaCl₂(aq) -> 2 NaCl(aq) + CaCO... - Organic Chemistry ANSWERED] Na₂CO3(aq) +CaCl₂(aq) -> 2 NaCl(aq) + CaCO... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/61173905-1656941477.49281.jpeg)