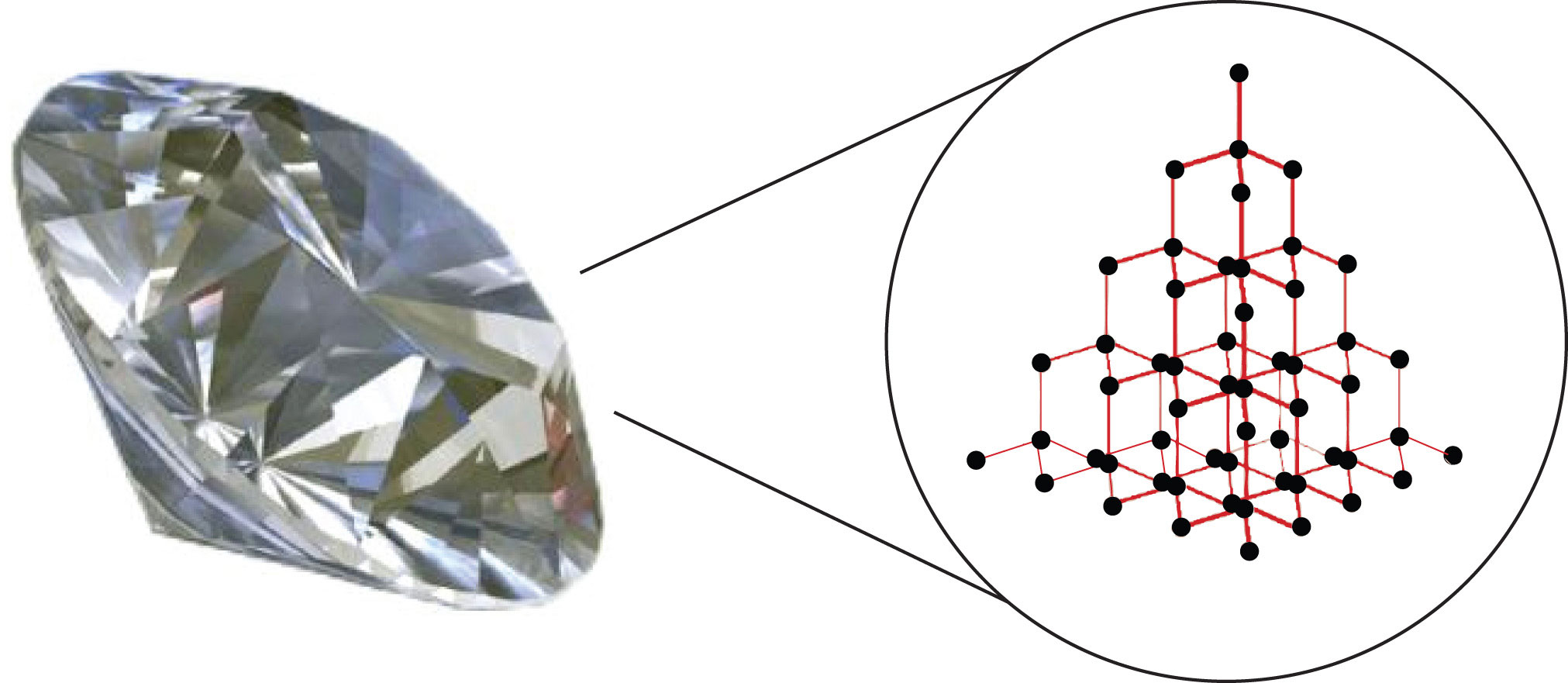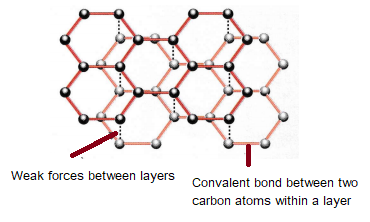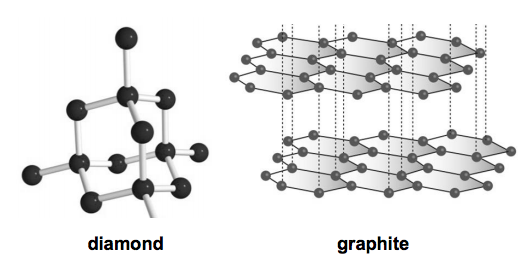
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry
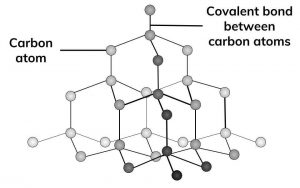
GCSE CHEMISTRY - What is the Structure of Diamond? - What is the Structure of Silicon? - GCSE SCIENCE.

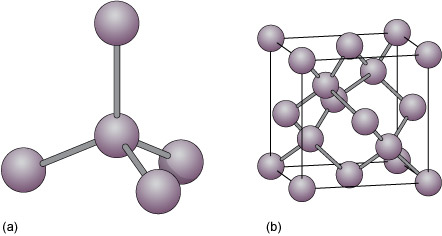
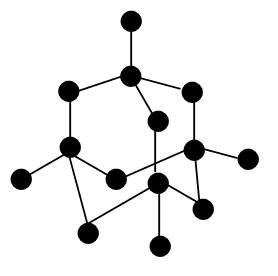
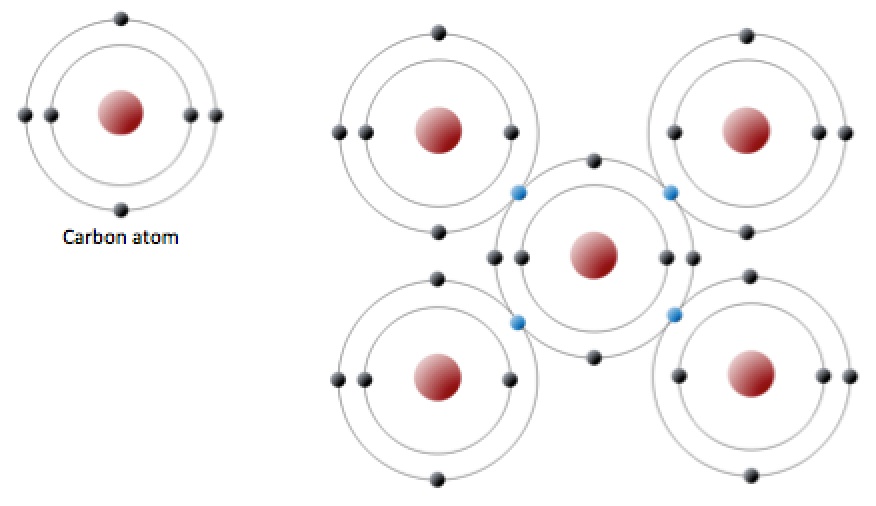
Nami || 15HINee on Twitter: "Let's talk diamonds💎 ✔️Diamonds are the hardest naturally occuring substance on earth. Why? Each carbon atom in diamond is covalently bonded to 4 other carbon atoms. Covalent

When non-metals combine together they share electrons to form molecules A covalent bond is a shared pair of electrons Non-metal + non-metal → Covalent. - ppt download

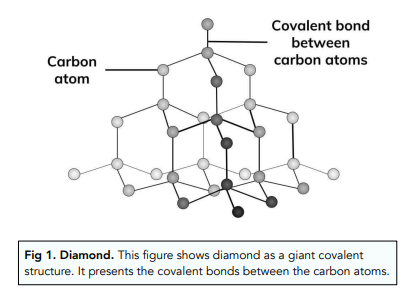
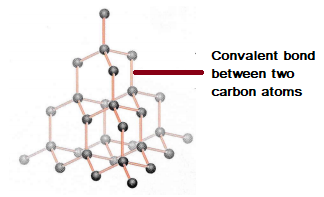
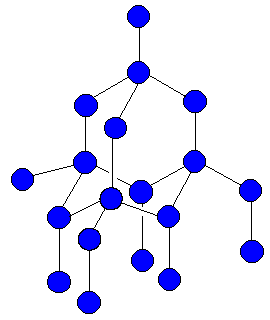
Describe the Structure of Diamond. Draw a Simple Diagram to Show the Arrangement of Carbon Atoms in Diamond. - Science | Shaalaa.com

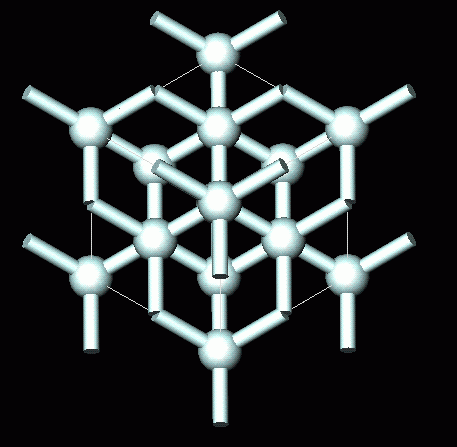
Crystallographic structure of diamond with tetrahedral bond angles of... | Download Scientific Diagram