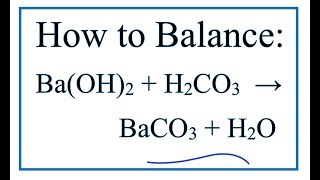
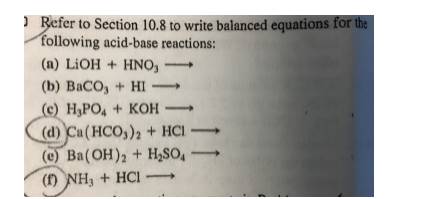
What are the balanced chemical and net ionic equations for the following reactions, solid barium carbonate and dilute sulphuric acid? - Quora

SOLVED: (4 points) Witherite is a mineral that contains barium carbonate. If a 1.68-g sample of witherite were to react completely with 49.2mLof0.2558 MHNO3, what would be the percent of barium carbonate

Chem Lab 100ML Barium standard solution (Plasma HIQU)(1.44 g BaCO3 / l 2% HNO3) Products | Fisher Scientific

Process Intensification in Nitric Acid Plants by Catalytic Oxidation of Nitric Oxide | Industrial & Engineering Chemistry Research
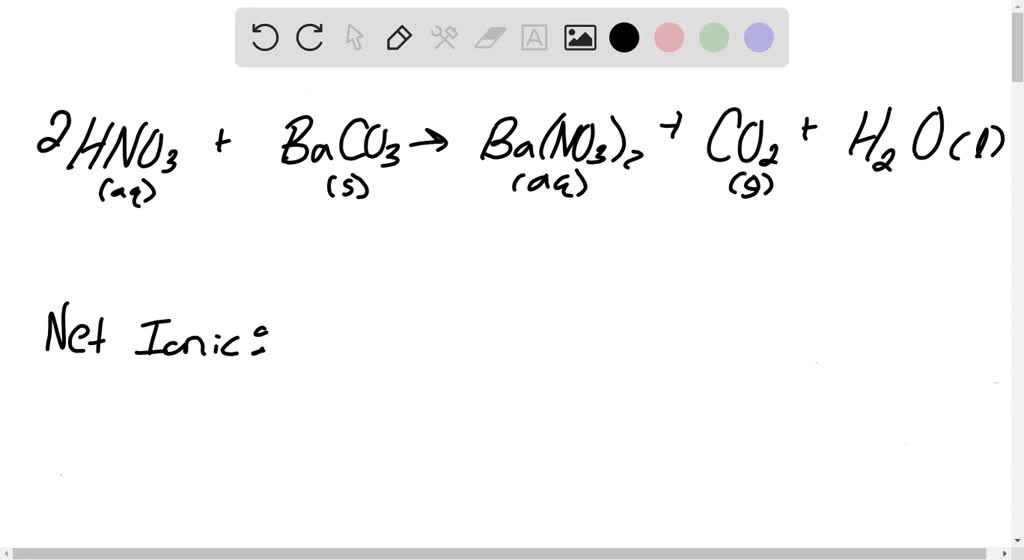
![ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry](https://media.kunduz.com/media/answer/raw/20220422054128757632-4413054.jpg?type=wm)






![ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/75663599-1659635277.7061477.jpeg)