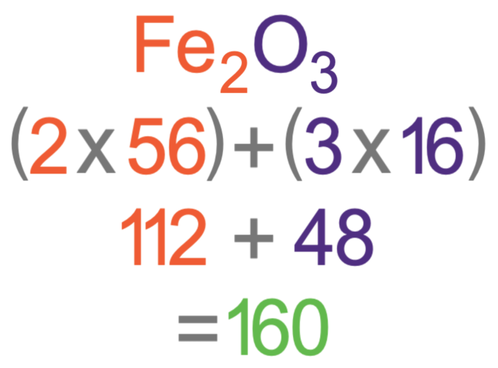Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively.

Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
How to answer this question? The formula of the iron oxide is Fe2O3. What is the maximum mass of iron that can be obtained from 240 tonnes of iron oxide, Fe2O3 (relative

SOLVED: Focus 1 Complete the diagram shown in Figure 5.1 to work out the formula mass of the iron oxide in the ore magnetite. (Ar: Fe=56, O=. 16.) Then use the following

Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that molecular mass is 159.69g.

T5 - Amountsof Sub - SLOP Bookletv 1 - No Sheets - Formula Literacy Write the formula for the - Studocu

definition mole explained molar mass mol mols calculations how to read equations in moles deducing equations from reacting mole ratios questions gcse chemistry igcse KS4 science A level GCE AS A2 O


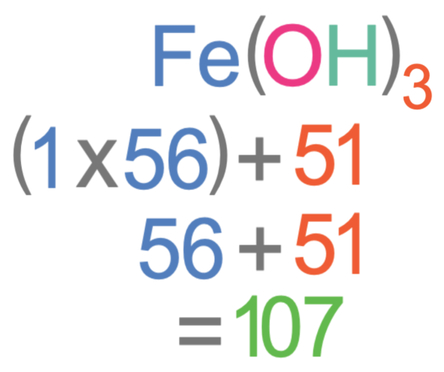






.PNG)