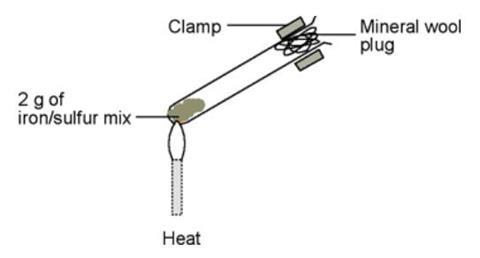
Iron(III)-Induced Activation of Chloride and Bromide from Modeled Salt Pans | The Journal of Physical Chemistry A

Write word equations and then balanced equations for the reaction taking place when:(a) Dilute sulphuric acid reacts with zinc granules.(b) Dilute hydrochloric acid reacts with magnesium ribbon.(c) Dilute sulphuric acid reacts with

Iron + Water = Rust | Chemistry! | Balancing Equations, Coefficients, Molecules - Science Q&A - YouTube

Question Video: Recalling the Products of the Reaction between Iron Metal and Dilute Mineral Acids | Nagwa

Causes chemistry of rusting rust prevention introduction to oxidation reduction REDOX reactions gcse igcse KS4 science chemistry revision notes revising
Give balanced chemical equations for the reaction of water with (a) Sodium (b) Iron - Sarthaks eConnect | Largest Online Education Community
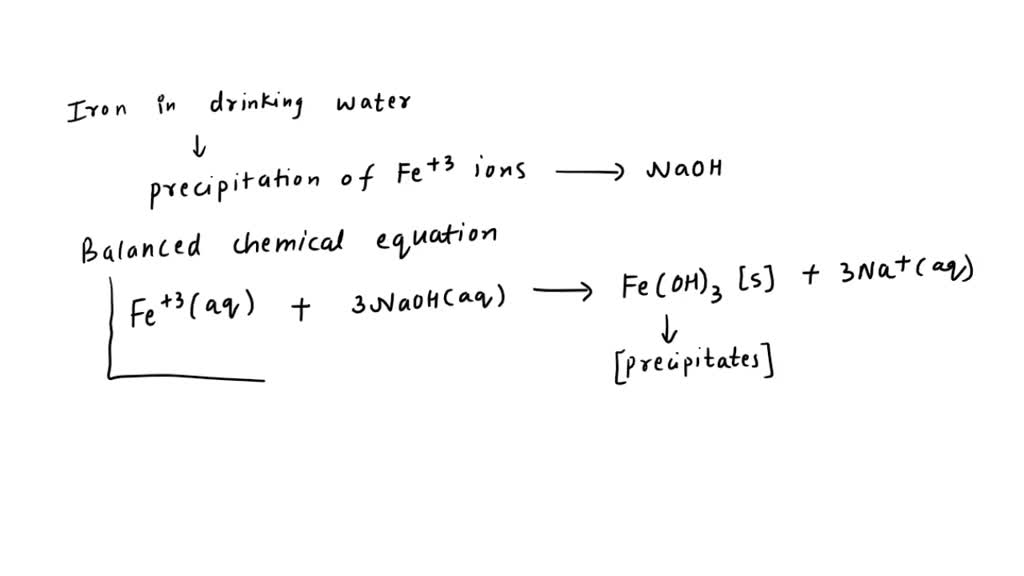
SOLVED: Iron in drinking water is removed by precipitation of the Fe3+ ion by reaction with NaOH to produce iron(III) hydroxide. Write the balanced chemical equation and the net ionic equation for