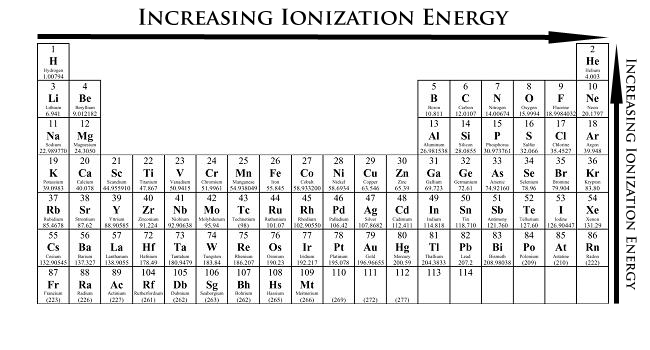
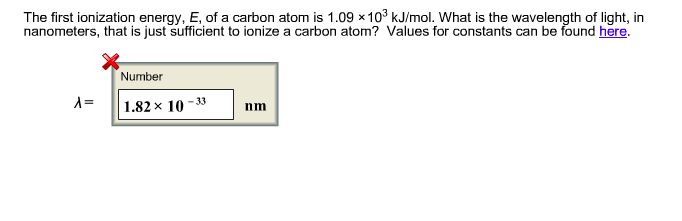
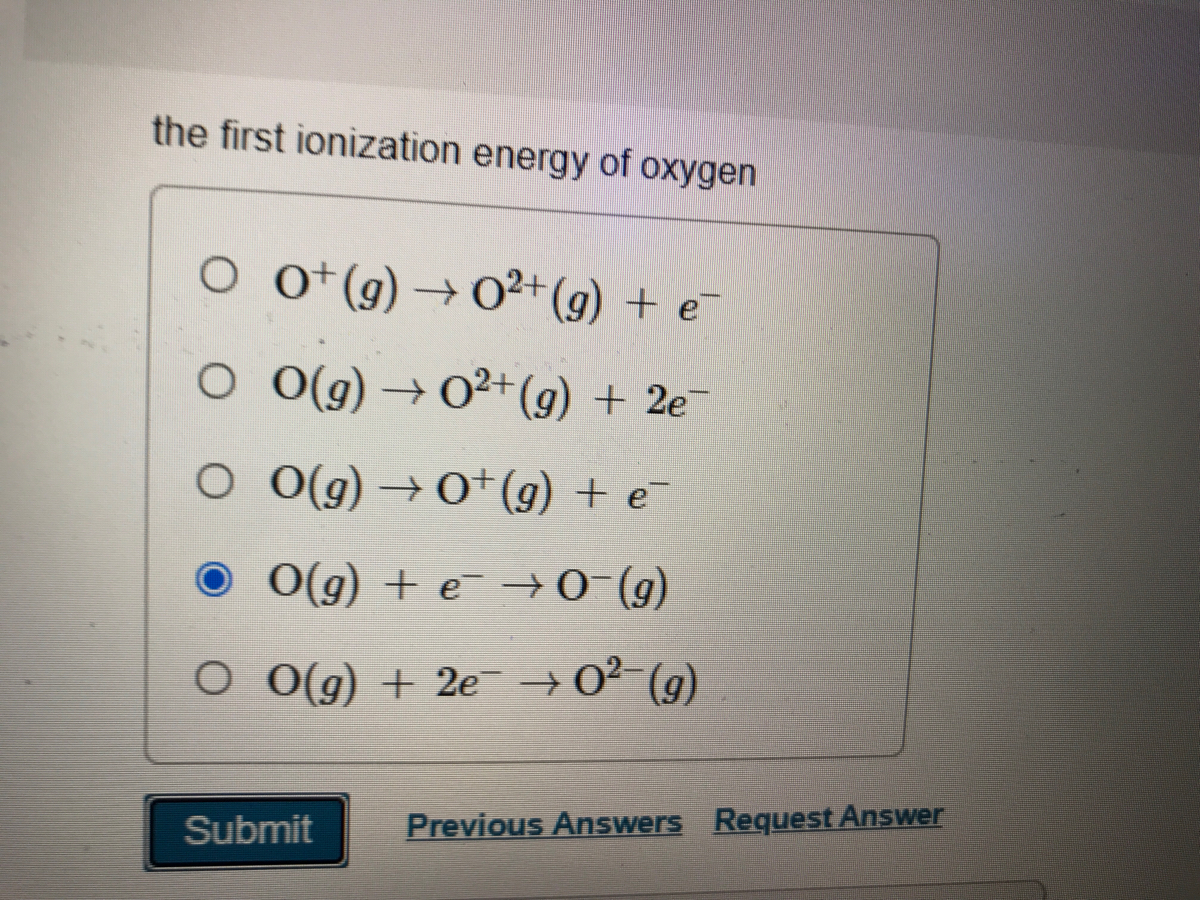
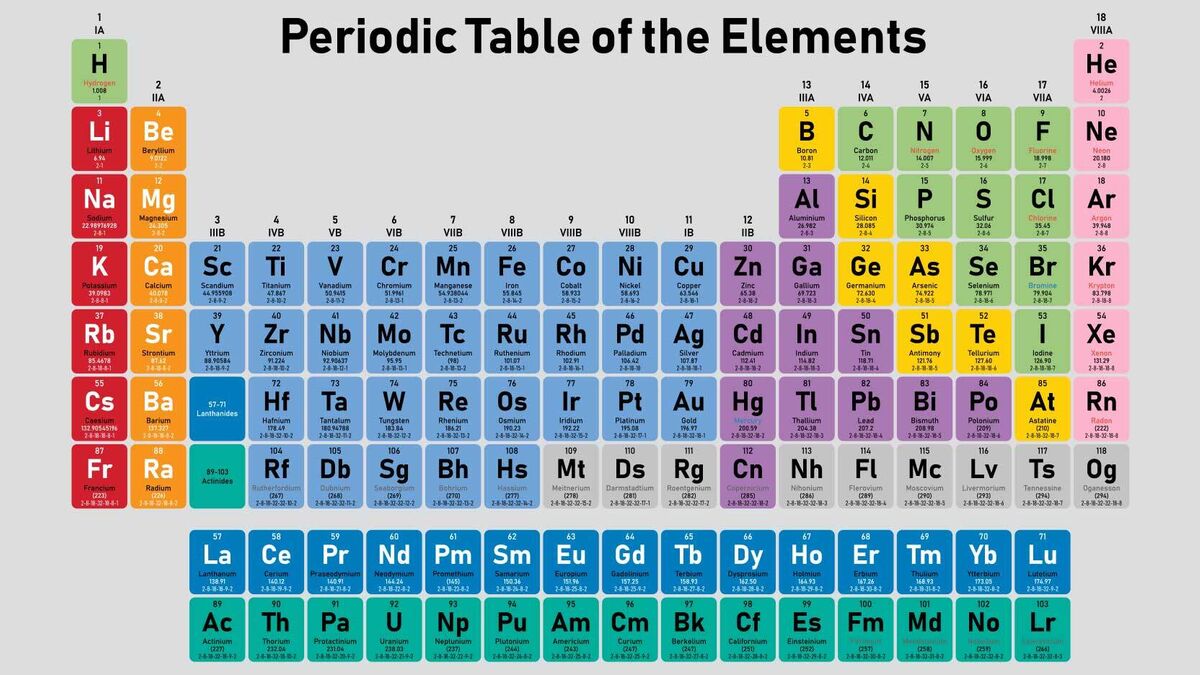
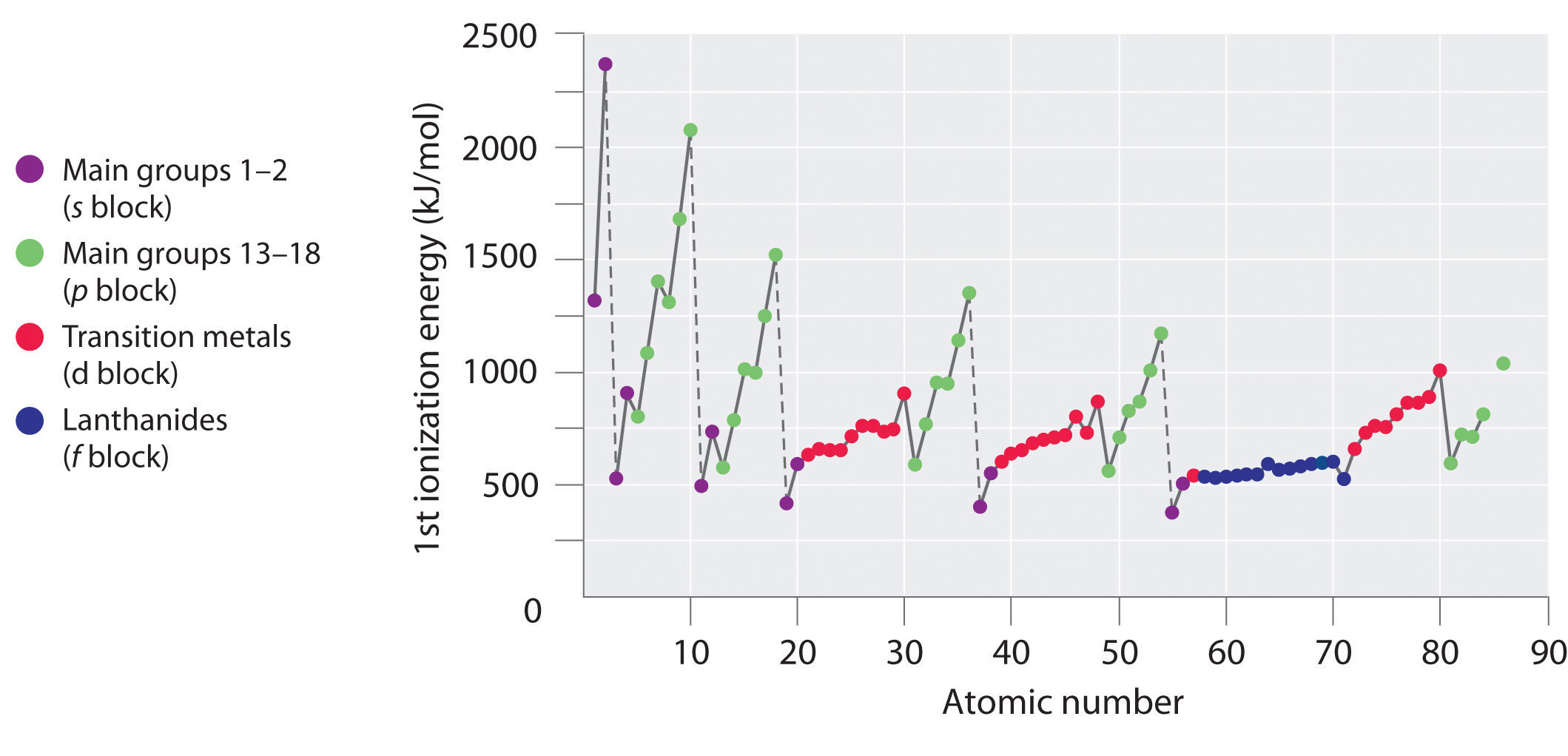
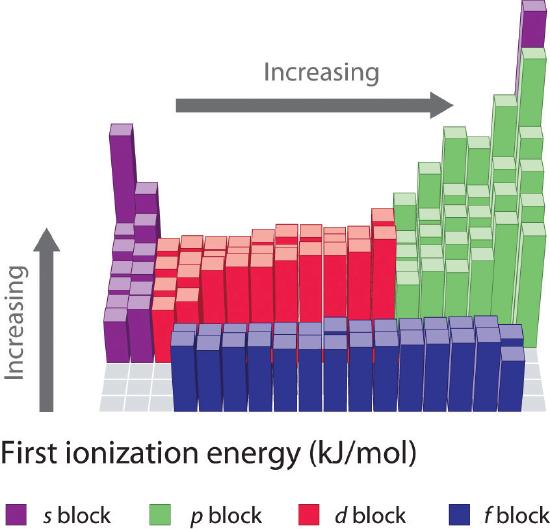
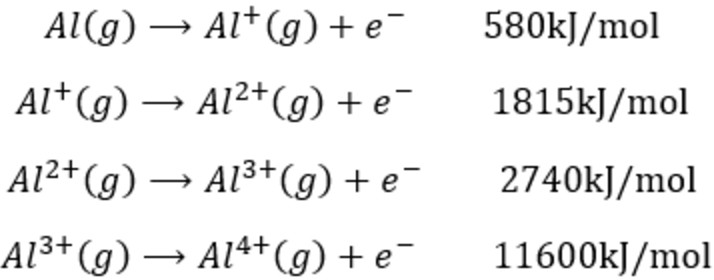The first ionisation enthalpy of carbon atom is greater than that of boron whereas the reverse is true for - Brainly.in

The correct order of the second ionisation potential of carbon, nitrogen, oxygen and fluorine is - YouTube

Carbon Chemical Element First Ionization Energy Stock Vector (Royalty Free) 1193986345 | Shutterstock
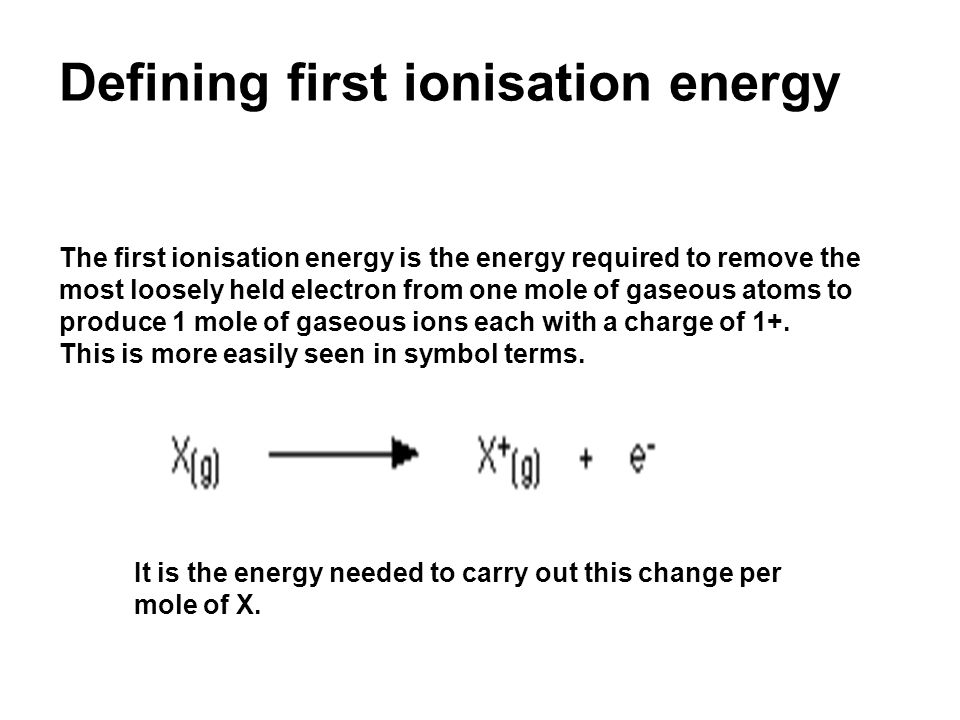
Defining first ionisation energy The first ionisation energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms. - ppt download

Premium Vector | Carbon chemical element with first ionization energy atomic mass and electronegativity values on scientific background
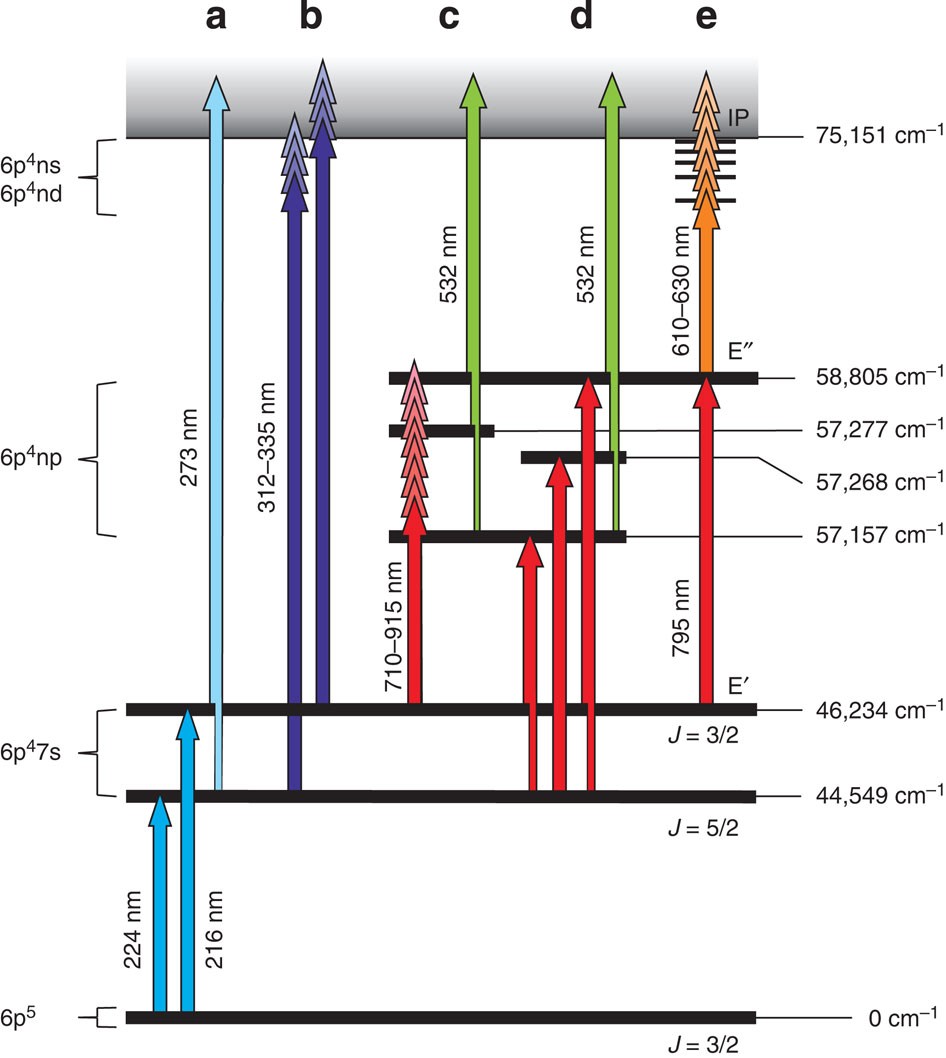
Measurement of the first ionization potential of astatine by laser ionization spectroscopy | Nature Communications