Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect
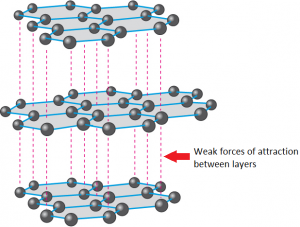
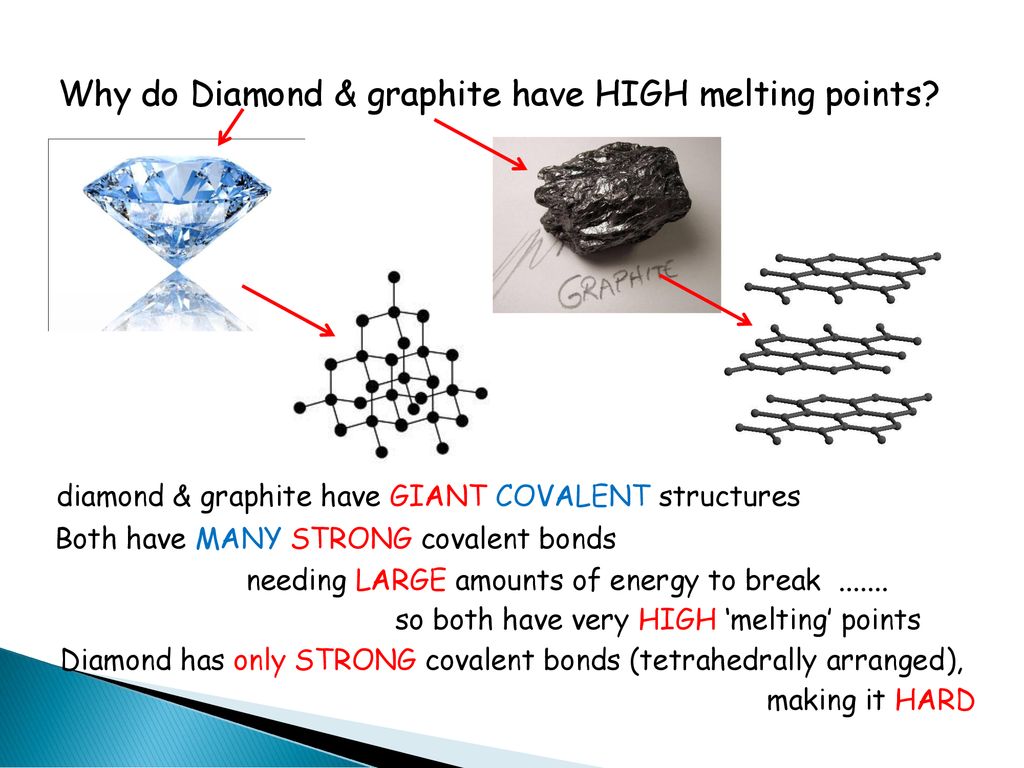
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

Carbon under extreme conditions: Phase boundaries and electronic properties from first-principles theory | PNAS

Equations of state at 4000K for the liquid, graphite, and diamond. The... | Download Scientific Diagram